1.10 Separation Techniques
Separation Techniques:
Filtration:
Consists of a barrier that one component of the mixture cannot pass through
e.g. water goes through rocks are caught

Distillation
One substance is evaporated off, the other is left in the flask
e.g. salt water is heated, water evaporates, salt is left

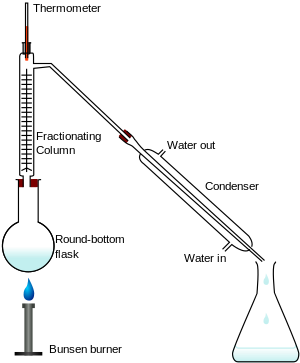
Fractional distillation
The mixture is evaporated and rises up the tube.
Different substances have different boiling points and so will evaporate at different times. As mixtures travel up the tube they condense and are collected.
e.g. different liquids
Crystallization
A solution is heated allowing the solvent to evaporate, the solute is left behind.
e.g. salt dissolved in water

Chromatography
Chromatography paper is placed in a solvent, different substances are placed on the paper, the different components of the substances will travel to different places.

Filtration:
Consists of a barrier that one component of the mixture cannot pass through
e.g. water goes through rocks are caught

Distillation
One substance is evaporated off, the other is left in the flask
e.g. salt water is heated, water evaporates, salt is left
Fractional distillation
The mixture is evaporated and rises up the tube.
Different substances have different boiling points and so will evaporate at different times. As mixtures travel up the tube they condense and are collected.
e.g. different liquids

Crystallization
A solution is heated allowing the solvent to evaporate, the solute is left behind.
e.g. salt dissolved in water
Chromatography
Chromatography paper is placed in a solvent, different substances are placed on the paper, the different components of the substances will travel to different places.
Comments
Post a Comment